-
Europe Endoscopic Retrograde Cholangiopancreatography (ERCP) Market is expected to grow from USD 490.6 million in 2024 to around USD 776.2 million by 2030, reflecting a 7.9% CAGR for 2025–2030.
Request Free Sample Report:https://www.stellarmr.com/report/req_sample/Europe-Endoscopic-Retrograde-Cholangiopancreatography-Market/738
Market Estimation, Growth Drivers & Opportunities
According to market research, Europe’s ERCP market was valued at USD 490.6 million in 2024, with growth projected at 7.9% CAGR, to reach approximately USD 776.2 million by 2030 .
Growth drivers include:
-
Rising prevalence of biliary and pancreatic disorders—such as gallstones, cholangitis, pancreatitis, and pancreatic cancer—among aging European populations.
-
Ongoing governmental and institutional investment in training and accreditation, notably through the European Society of Gastrointestinal Endoscopy (ESGE), which has introduced formal ERCP certification and training mandates.
-
Advances in technology—high-definition imaging systems, safer disposable duodenoscopes, and AI-enhanced visualization platforms—boost procedural safety and adoption rates .
Opportunities include accelerating adoption of single-use scopes to address infection concerns, integrating AI diagnostics in visualization systems, expanding ERCP services into under-served regions, and offering low-cost consumable options in high-penetration countries.
U.S. Market: Not Applicable
This press release is focused on the European region; U.S. market details are not included.
Market Segmentation: Largest Segment
Based on segmentation data:
-
By Product:
-
Endotherapy devices (stents, sphincterotomes, guidewires, balloons) held the largest share (≈38–40%) of product revenue in 2024 .
-
Endoscopes (diagnostic imaging systems) represent the fastest-growing segment, gaining traction for AI-ready hardware and disposable formats .
-
-
By Procedure/Application:
-
Biliary sphincterotomy and stenting dominate procedural volume due to high rates of biliary obstruction cases. Pancreatic procedures are also rising gradually.
-
-
By End‑User:
-
The bulk of ERCP procedures are conducted in hospitals and gastroenterology clinics. Ambulatory surgical centers (ASCs) are emerging but represent a smaller share.
-
Competitive Analysis: Top 5 Companies
Leading companies active across Europe include:
-
Olympus Corporation (Japan) – Holds a leading global share in endoscopy systems and ERCP hardware, with strong presence in Europe through direct subsidiaries and hospital contracts .
-
Karl Storz SE & Co. (Germany) – Provides high-end flexible and rigid endoscopes and visualization systems across major European markets with deep distribution networks .
-
FUJIFILM Holdings Corporation (Japan) – Offering advanced imaging and therapeutic ERCP systems, expanding footprint in Western Europe
-
Boston Scientific Corporation (USA) – Supplies endotherapy devices (stents, sphincterotomes, guidewires) widely used in European ERCP procedures
-
Ambu A/S (Denmark) – Pioneered single-use duodenoscope systems gaining traction in Europe due to infection-control priorities.
Other key players include Medtronic PLC, Steris PLC, ConMed Corporation, Johnson & Johnson surgical technologies, and Cook Medical, all investing in disposable devices, AI, and training partnerships .
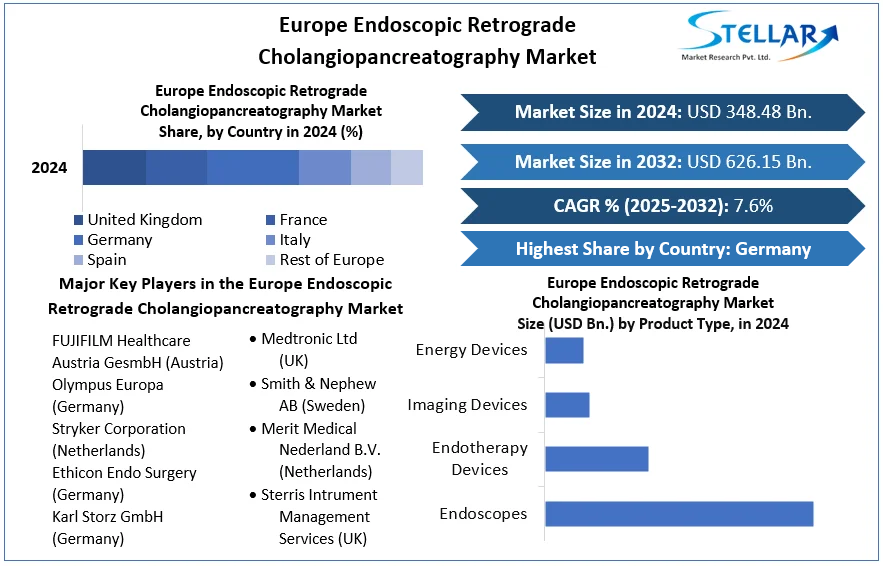
Regional Analysis: Germany, UK, France, Spain, Italy
-
Germany: Led the European ERCP market in 2024 with USD 117.5 million, forecast to reach USD 176.4 million by 2030 at 6.9% CAGR. Endotherapy devices led product share, while endoscopes are fastest-growing
-
United Kingdom & France: Both countries contribute significantly to the region’s share. The UK market is expected to grow at ~7.5% CAGR; France at ~9.2% over 2022–2028, driven by the rising incidence of GI cancers and increased procedural penetration .
-
Spain: Generated USD 37.3 million in revenue in 2024, projected to reach USD 59.2 million by 2030 at 7.9% CAGR. The product and procedure segmentation in Spain mirrors broader European trends
-
Italy and Others: Italy and other Western European countries contribute with growing adoption of ERCP devices in tertiary hospitals. Eastern Europe and Nordic countries show rising growth but from lower bases.
Across Europe, countries like Germany, UK, and France dominate due to advanced healthcare infrastructure, high procedural volumes, and strong payer systems encouraging technological investment
Conclusion
Europe’s ERCP market is projected to grow from USD 490.6 million in 2024 to approximately USD 776.2 million by 2030, with a 7.9% CAGR Growth is driven by increasing prevalence of pancreaticobiliary diseases, healthcare system modernization, and technology adoption including single-use scopes and AI-enabled imaging.
Key growth opportunities:
-
Scaling single-use disposable duodenoscopes, particularly for infection control and cross-border regulatory compliance.
-
Incorporating AI-assisted visualization and analytics to improve procedural accuracy and training outcomes.
-
Expanding ERCP access into secondary-tier cities and ambulatory centers to reach underserved populations.
-
Launching cost-effective consumable and service-based models to increase affordability and volume.
-
Supporting professional training and ESG compliance, aligning with ESGE accreditation standards and European Medical Device Regulation.
About us
-
-
Phase 3,Navale IT Zone, S.No. 51/2A/2,
Office No. 202, 2nd floor,
Near, Navale Brg,Narhe,
Pune, Maharashtra 411041
+91 9607365656
sales@stellarmr.com
Author
rushistellar